| # | Task | What it involves (definition) | Purpose / Why it matters |
|---|---|---|---|
| 1 | Post‑approval tasks | Version‑control consent docs, build site‑initiation checklist, schedule first recruitment. | Ensures ongoing compliance and smooth transition to data collection stage. |
| 2 | Secure final IRB approval | Receive and file the stamped consent forms and official approval letter. | Mandatory before any human‑subject interaction; unlocks FWA filing and study start. |
| 3 | Respond to IRB clarifications | Address reviewer questions, revise documents, and resubmit with tracked changes. | Typical 1–2 cycles; must be completed for final approval. |
| 4 | Pre‑review quality check | Internal compliance team or mentor reviews packet for template errors and missing signatures. | Prevents needless IRB deferrals and time loss. |
| 5 | Package initial IRB application | Upload protocol, consent/assent forms, worksheets, and pay fee in the IRB portal. | Kicks off the formal IRB review clock. |
| 6 | Reliance agreement draft | Prepare SMART IRB or institutional reliance template if external sites/cores are involved. | Enables single‑IRB review and clarifies oversight responsibilities. |
| 7 | COI disclosures | Submit signed financial and intellectual conflict‑of‑interest statements for all key personnel. | Confirms no undisclosed relationships that could bias the research. |
| 8 | Investigator credentials & CITI certs | Compile PI/co‑investigator CVs, licenses, and CITI human‑subjects training proofs. | Shows the team is qualified and ethics‑trained. |
| 9 | Safety monitoring plan | Outline adverse‑event definitions, reporting timelines, oversight personnel, and stopping rules. | Even minimal‑risk studies need a documented escalation path. |
| 10 | Data‑management & confidentiality plan | Describe REDCap roles, encryption, ID codes, storage duration, and destruction timeline. | Demonstrates robust data‑security safeguards, critical for work with children. |
| 11 | Recruitment materials | Draft flyers, e‑mails, social posts, and a phone‑screen script exactly as they’ll appear. | IRB must approve every word shown to potential participants to avoid coercion. |
| 12 | HIPAA authorization clause | Add standalone page or section spelling out PHI access, storage, and data‑sharing terms. | Ensures HIPAA compliance and transparency on privacy. |
| 13 | Create adolescent consent form | Separate form with teen‑friendly language for ages 14‑17 (optional but often requested). | Respects emerging autonomy of older minors and streamlines signatures. |
| 14 | Draft child assent form | Create a simplified, age‑appropriate version (visuals, plain language) for younger children. | Federal regulations require assent when children can understand. |
| 15 | Develop parent/guardian consent | Turn protocol info into an 8th‑grade‑level consent document covering risks, benefits, rights, contacts, and HIPAA text. | Legally required permission because participants are minors. |
| 16 | Draft full study protocol | Write the complete background, objectives, methods, inclusion/exclusion, statistics, and safety sections. | Core document every other material references; IRB approval hinges on it. |
| 17 | Define final study synopsis | Draft a one‑page snapshot of study population, aims, endpoints, and procedures. | Gives IRB reviewers a quick roadmap before they open the full protocol. |
IRB & Ethics Packet
IRB & Ethics Packet
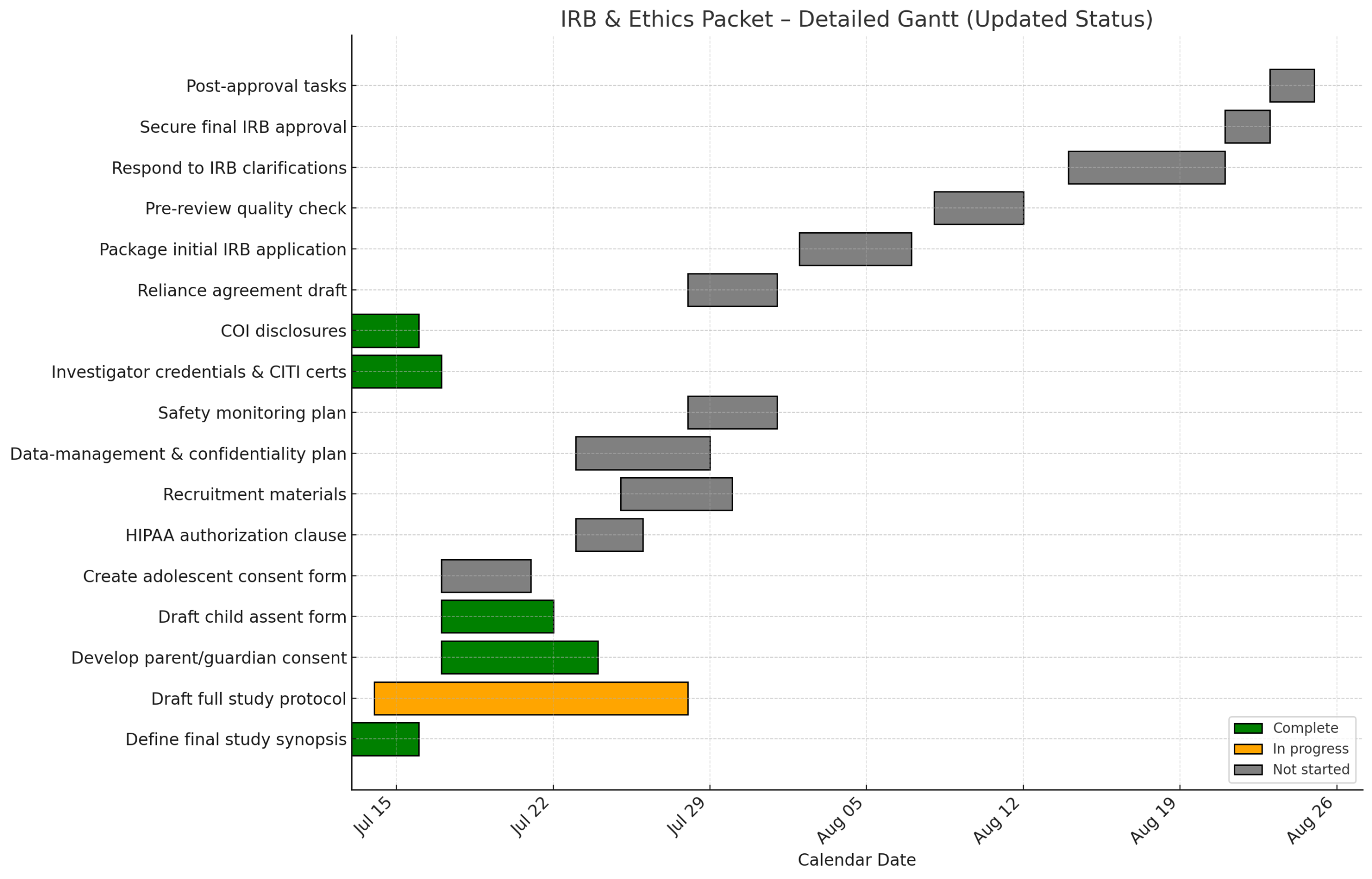
Milestone checklist (July 2025 → approval)
- 1. Post‑approval tasks Not Started
- 2. Secure final IRB approval Not Started
- 3. Respond to IRB clarifications Not Started
- 4. Pre‑review quality check Not Started
- 5. Package initial IRB application Not Started
- 6. Reliance agreement draft Not Started
- 7. COI disclosures ✓ Complete
- 8. Investigator credentials & CITI certs ✓ Complete
- 9. Safety monitoring plan Not Started
- 10. Data‑management & confidentiality plan Not Started
- 11. Recruitment materials Not Started
- 12. HIPAA authorization clause Not Started
- 13. Create adolescent consent form Not Started
- 14. Draft child assent form ✓ Complete
- 15. Develop parent/guardian consent ✓ Complete
- 16. Draft full study protocol In Progress
- 17. Define final study synopsis ✓ Complete
IRB Protocol Outline: RESET Study
1. Study Purpose
The Resilience & Epigenetic Signatures of Early‑Life Trauma (RESET) Study aims to quantify how cumulative childhood adversity accelerates biological aging—and to test whether psychosocial resilience factors and trauma‑informed therapy can slow or reverse that acceleration. We will employ a multi‑clock epigenetic approach in children, adolescents, and young adults.
2. Study Endpoints and Hypotheses
2.1 Primary Endpoints
- Age‑Acceleration (H1): We hypothesize that participants with high cumulative ACE scores (≥ 4) will exhibit at least 1.5 years of epigenetic age acceleration—averaged across multiple clocks—relative to chronological age, compared with low‑ACE controls (≤ 1).
- Therapy Effect (H2): Among high‑ACE participants, those completing at least 12 sessions of trauma‑informed cognitive behavioral therapy (CBT) will demonstrate a 25 % or greater reduction in the annual rate of epigenetic‑clock progression over 24 months, compared to wait‑list controls.
2.2 Secondary and Mechanistic Endpoints
- Buffering by Sleep (H2a): We expect that participants in the top tertile of actigraphy‑measured sleep efficiency will show a 40 % attenuation of the ACE → age‑acceleration relationship, modeled as an ACE × sleep interaction in a linear mixed model.
- Inflammatory Amplification (H2b): We anticipate that elevated baseline CRP (> 3 mg/L) will amplify the ACE → acceleration association by at least 0.3 standard deviations, tested via an ACE × CRP interaction term.
- Clock Concordance (H3): We will compare the sensitivity of DunedinPACE versus GrimAge to therapy‑induced change, expecting a mean paired difference greater than 0.3 years (p < 0.05) using repeated‑measures ANOVA.
2.3 Null Hypotheses
- H0‑1: No difference in baseline epigenetic age acceleration between high‑ and low‑ACE groups.
- H0‑2: Trauma‑informed therapy does not alter the annual rate of epigenetic aging compared to wait‑list controls.
3. Study Design and Methods
3.1 Overview of Specific Aims
| Aim | Question | Read‑outs |
| 1 | Baseline association | PedBE clock (age 8–17); GrimAge & DunedinPACE (age 18–35); telomere length |
| 2 | Moderators & mediators | Actigraphy (sleep & activity), social‑support scales, serum CRP & IL‑6 |
| 3 | Longitudinal change over 24 months | Change in clock reading per year between baseline and Month 24 |
3.2 Participants
We will enroll 400 participants aged 8–35, stratified by ACE score (low: 0–1; high: ≥ 4). Inclusion criteria include the ability to assent or consent and absence of severe medical comorbidity, active substance dependence, or cognitive impairment that would preclude study participation.
3.3 Procedures
Participants will visit at baseline (V0), Month 6 (V1), Month 12 (V2), Month 18 (V3), and Month 24 (V4). Children aged 8–17 will provide buccal swabs for the PedBE clock; adults aged 18–35 will undergo blood draws for GrimAge and DunedinPACE assays, with a subset sampled for telomere qPCR. All participants will complete the standardized ACE questionnaire and social‑support scales, and wear an actigraphy device for seven consecutive days at each visit.
For Aim 3, high‑ACE participants will be randomized 1:1 to receive 12 sessions of trauma‑informed CBT plus a home sleep/activity optimization module or to a wait‑list control group (offered therapy after Month 24).
3.4 Data Analysis Plan
- Aim 1: Two‑sample t‑tests will compare high‑ versus low‑ACE participants on baseline epigenetic-age acceleration.
- Aim 2: Linear mixed models will assess interaction effects between ACE score and sleep efficiency or CRP levels.
- Aim 3: Two‑sample t‑tests and ANCOVA will evaluate differences in clock change rates, supplemented by repeated‑measures ANOVA for cross‑clock sensitivity. All analyses will adjust for covariates including age, sex, race/ethnicity, estimated cell‑type composition, and assay batch effects.
4. Risks and Benefits
4.1 Risks
- Biospecimen Collection: Blood draws may cause transient discomfort, bruising, or fainting. Buccal swabs pose minimal risk.
- Psychological Distress: Discussing adverse childhood experiences may elicit emotional discomfort. Participants may skip any question or pause participation at any time.
- Confidentiality Breach: Handling sensitive personal data carries a small risk of unauthorized disclosure.
Risk Mitigation: Licensed phlebotomists will perform blood draws. A trained counselor will debrief and provide support following the ACE questionnaire. Data will be de‑identified and stored on encrypted, HIPAA‑compliant REDCap servers with role‑based access controls.
4.2 Benefits
Participants may gain insights into their stress physiology and epigenetic health. High‑ACE individuals randomized to the intervention may experience improved coping and resilience. The broader scientific community and future patients will benefit from improved understanding of how psychosocial therapies can modulate biological aging.
5. Data Management and Privacy
All participant data will be entered into a secure REDCap database. Identifiers will be replaced with study codes; the key linking codes to identities will be stored separately on an encrypted drive accessible only to the PI. Data transfers and storage will comply with institutional policies and HIPAA regulations.
6. Safety Monitoring
The PI will oversee all adverse events. Any serious adverse event (SAE) will be reported to the IRB within 24 hours. A Data and Safety Monitoring Board (DSMB) will review interim safety reports semi‑annually.
7. Timeline
- Month 0–3: IRB submission and approval
- Month 4–6: Recruitment and baseline visits
- Month 7–30: Follow‑up visits and intervention delivery
- Month 31–36: Data analysis and manuscript preparation
Prepared by:
Dr. Stephen C. Rose , RESET Study Principal Investigator
[Institution Name], [Date]
Dr. Adam O’Brian, RESET Study CO-PI
